Fast to Clinic
- mAb Fast CMC: 6 months from Sequence to Tox batch release
- bsAb Fast CMC: As fast as 7 months from Sequence to Tox batch release
ProBoxTM Process Development Tool Box
- Solutions for challenging problems with our expertise on the knowledge of protein structures and quality attributes
Customized Solutions for mAb,BsAb,Protein
- 3 Processes: fed-batch, high density inoculation and perfusion process according to different molecules
- Analyticial & bioassay method development for mAb,bsAb,protein
Host Cell Commercial License
- Wide type CHOK1-GenS and ADCC enhanced CHOK1-ADCC+ cell license to be chosen
- Combo license at discounted price
- Royalty free
IND-enabling CMC Development Platform
-

mAb Fast CMC
For mAb, Naked Ab of ADC6 months from Sequence to Tox batch release
-

bsAb Fast CMC
For bsAbAs fast as 7 months from Sequence to Tox batch release
-

Intensified CMC
For bsAb, Protein9 months from Sequence to Tox batch release
√ 6 months from sequence to Tox DS release
√ 12 months from sequence to IND
√ 0.5kg/200L, 1.2kg/500L GMP DS is guaranteed to deliver

Applicable to symmetrical bispecific antibody:
√ 7 months from sequence to Tox DS release
√ 13 months from sequence to IND

Applicable to asymmetrical bispecific antibody:
√ 8.5 months from sequence to Tox DS release
√ 14.5 months from sequence to IND

Intensified CMC Highlights
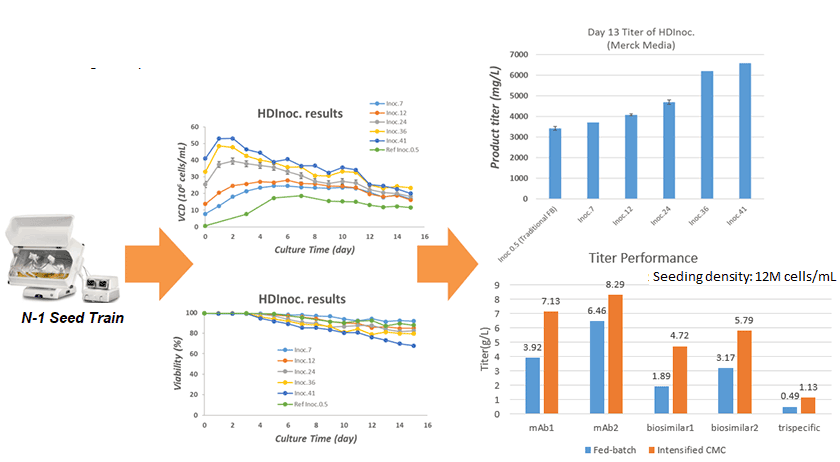
- High density inoculation technology
- Easy to have 30%-150% titer improvement compared to standard CMC
- 30%-50% manufacturing cost down per gram of Ab
- Friendly differential pricing according to titer improvement
| Seed Train | Upstream Process | Features | Culture Longevity (Seed-Harvest) | Max VCD | Typical Titer | |
|---|---|---|---|---|---|---|
| Standard CMC |  Conv. Seed Train |
 Conv_FB Conv_FB |
|
25-30 Days | 20-30 M/mL | 3-5 g/L |
| Intensified CMC |  Conv. banking N-1 Per. Seed Train |
 HDInoc_FB HDInoc_FB |
|
30-35 Days | 30-50 M/mL | 7-9 g/L |
Intensified CMC Significantly Brings Titer Improvement
80% projects titer increase between 80%-150%
√ ProBoxTM -process development tool box is build based on ProBio’s ~100 CMC project experience and it classifies, summarizes and integrates ~200 technical challenges and solutions in process development and analytical development.
√ The ProBoxTM is updated in real time to help process researchers better cope with new process development challenges.
√ In addition, ProBoxTM analyzes multiple types of bispecific antibodies and proteins and summarizes the challenges and solutions based on the understanding of their structure and quality attributes.

Versatile Analytical Procedures
- General properties: UV280, AAA, pH, Osmolality, Color, Clarity, etc.
- Structural characterization: LC-MS, CD, DLS, DSC, etc.
- Product-related impurities: SEC/CEX/HIC/RP-HPLC, CE-SDS, icIEF, etc.
- Process-related impurities: HCDNA, HCP, rProteinA, Endotoxin, Bioburden, etc.
- Bioactivities: ELISA binding and Cell-based assay, ADCC, CDC, ADCP, MLR, Fc-binding
Strong Capability in Method Development
- Experience of >10 kinds of CMC biologics, including mAb, bsAb, tsAb, scFv, hILs, coagulation factors, protein complex, and many other specially designed molecules.
- Dedicated cell line engineering team to develop cell line based on target MOA
- Can start method development as early as possible (e.g. cell pool stage)
Hardware and Software
- Powerful instruments: 2 mass spectrometers (QE Orbitrap and Q-TOF), UHPLC systems of mainstream brands (Agilent/Waters/Thermo), CE systems (PA800 plus, Maurice, etc.), Microplate readers (Molecular Devices)
- Software meets compliance: Audit trails available, with GMP, GLP, and 21 CFR Part 11 compliance
Determine the Biological Potency of Broad Types of Targets
Cell-based assay Dev. Capability
- Dedicated cell line engineering team to develop cell line based on target MOA
- Experience of >30 targets, multiple off-the-shelf cell lines to support IND/BLA filing
- In compliance with ICH and USP to perform method development, optimization and validation.
- Clear background and traceability of cell line, which is compliant with authority regulation
Different kinds of targets
- mAb, bsAb, recombinant protein, Cytokine, T-cell engager
- Immune checkpoints, tumor-associated antigens, inflammation factors, cytokines, coagulation factors, GPCRs
- Anti-cell proliferation, apoptosis, T cell activation, cytokine release, neutralization, etc.
Platforms for characterization
- ADCC, CDC, ADCP, Mixed Lymphocyte Reaction
- Fc-binding
- FcγRIIIA (CD16a) 158V, FcγRIIIA (CD16a) 158F, FcγRIIA (CD32a) 131H, FcγRIIA (CD32a) 131R
- FcγRI (CD64), FcRn, C1q
Quotations and Ordering
To request a quotation, please contact us via our secure online messaging system.

